粉末消火設備は粉末消火薬剤を火源に大量放射することで、燃焼面を覆い空気遮断による窒息効果、粉末消火薬剤が熱により分解し、発生する炭酸ガスによって空気中の酸素濃度を下げる希釈窒素効果、化学作用によって燃焼の連鎖反応を中断する負触媒効果(抑制効果)の相乗効果により、即効的に消火できます。


粉末消火設備の特長
特長01
短時間で大量の消火薬剤を放出することにより強力な消火効果を得ることができます。
特長02
瞬時に消火しますので、燃焼拡散が速い危険物や指定可燃物火災の消火設備に最適です。
特長03
消火薬剤は気温に左右されず、性能に変化がありませんので寒冷地でも安心して使用できます。
特長04
電気絶縁性が優れていますから、変圧器など高圧電気設備にも使用できます。
特長05
貯蔵容器等の操作が簡単明瞭ですから、使用後、配管のクリーニングなども簡単にできて便利です。
粉末消火剤の種類と特長
| 主成分 | 特長 | 色 | 適合火災 | |||
|---|---|---|---|---|---|---|
| 一般火災(A) | 油火災(B) | 電気火災(C) | ||||
| 第1種粉末 | 炭酸水素ナトリウム | 180ミクロン以下の微粒子の炭酸水素ナトリウム(主成分)に、無水硅素、シリコンなどを添加したものです。 | 白あるいは 淡青色 | − | ○ | ○ |
| 第2種粉末 | 炭酸水素カリウム | 180ミクロン以下の微粒子です。 パープルKとも呼ばれています。 | 紫色 | − | ○ | ○ |
| 第3種粉末 | リン塩酸類 | リン酸二水素アンモニウムを主成分とし、180ミクロン以下の微粒子です。この微粒子一粒一粒の表面には吸湿を防ぐために、当社独自の製造方法による特殊コーティングがしてあります。また、滑剤としてホワイトカーボン、タルクなど数種類の成分を混入しています。その特色は次のようなものです。 混合してある数種類の安定剤は特に注意をはらっていますから、消火薬剤の固化、吸湿の防止に優れています。 粉末の微粒子を覆っているシリコンは最適条件で処理してありますから、撥水性に優れています。 A・B・Cの火災にすばらしい適消性があります。 | ピンク色 | ○ | ○ | ○ |
| 第4種粉末 | 炭酸水素カリウムと尿素 | この消火薬剤は「モネックス」といいます。英国IC社が開発したまったく新しい粉末消火薬剤で、炭酸水素カリウムと尿素による特殊化合物です。180ミクロン以下の微粒子で、特殊シリコン処理をした上数種類の滑剤を混入してあります。 炎にかけると熱によって一粒一粒がさらに分割して超微粒子になるため、活性表面積が増大し、強力な消化力を発揮します。石油製油工場・航空機発着場などの大型火災が予想される場所に適した消火剤といえるでしょう。 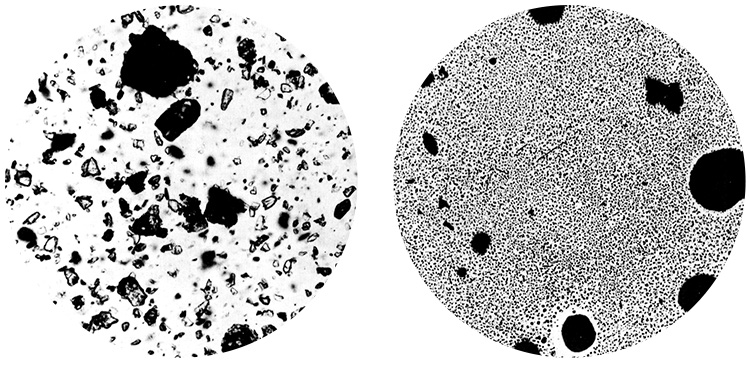 写真は、「モネックス」の粒子が火災に曝露後、どのように分解されたかを示すものです。粒子が微粒化され表面積が増大していることがわかります。 | ねずみ色 | − | ○ | ○ |
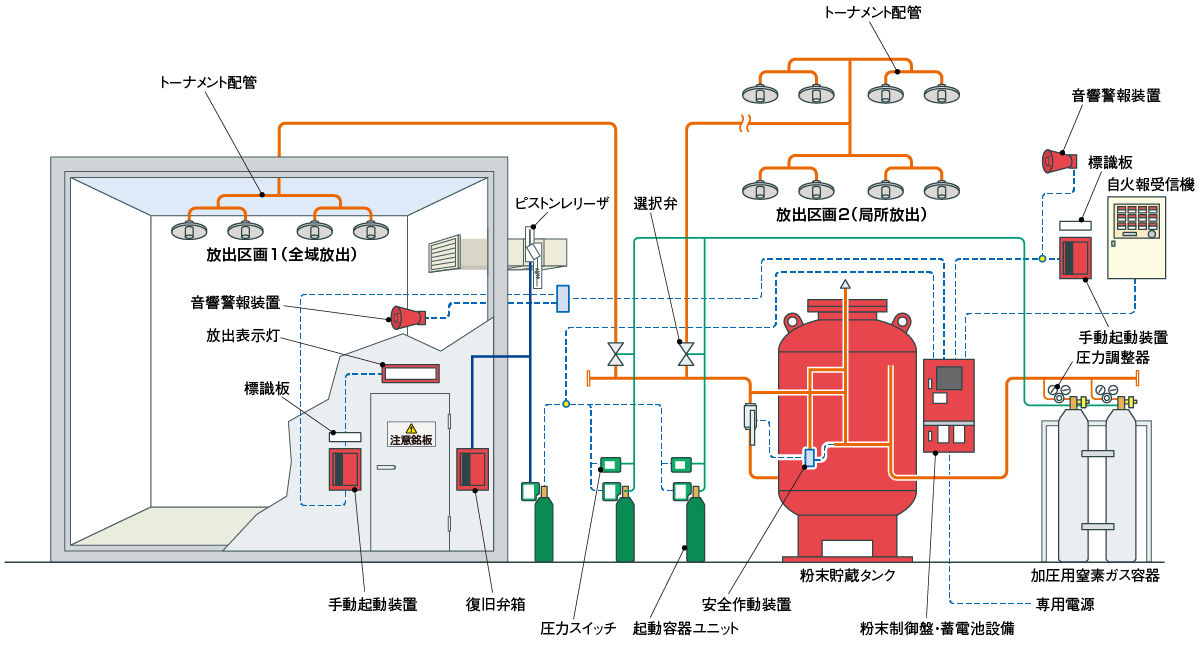
粉末消火設備のシステム構成図
粉末消火設備の種類
粉末モニター
石油・石油化学プラントなどの岸壁や桟橋・船舶、あるいは陸上の危険物施設・航空機格納庫などの消火活動を行う、大型の固定消火システム。

移動式粉末消火設備
屋上駐車場、修理工場など小規模の火災の消火に用いられます。

関連情報
容器弁点検
粉末消火設備は、設置後30年までに、容器弁の耐圧性能検定を終わらせることが義務付けられています。
